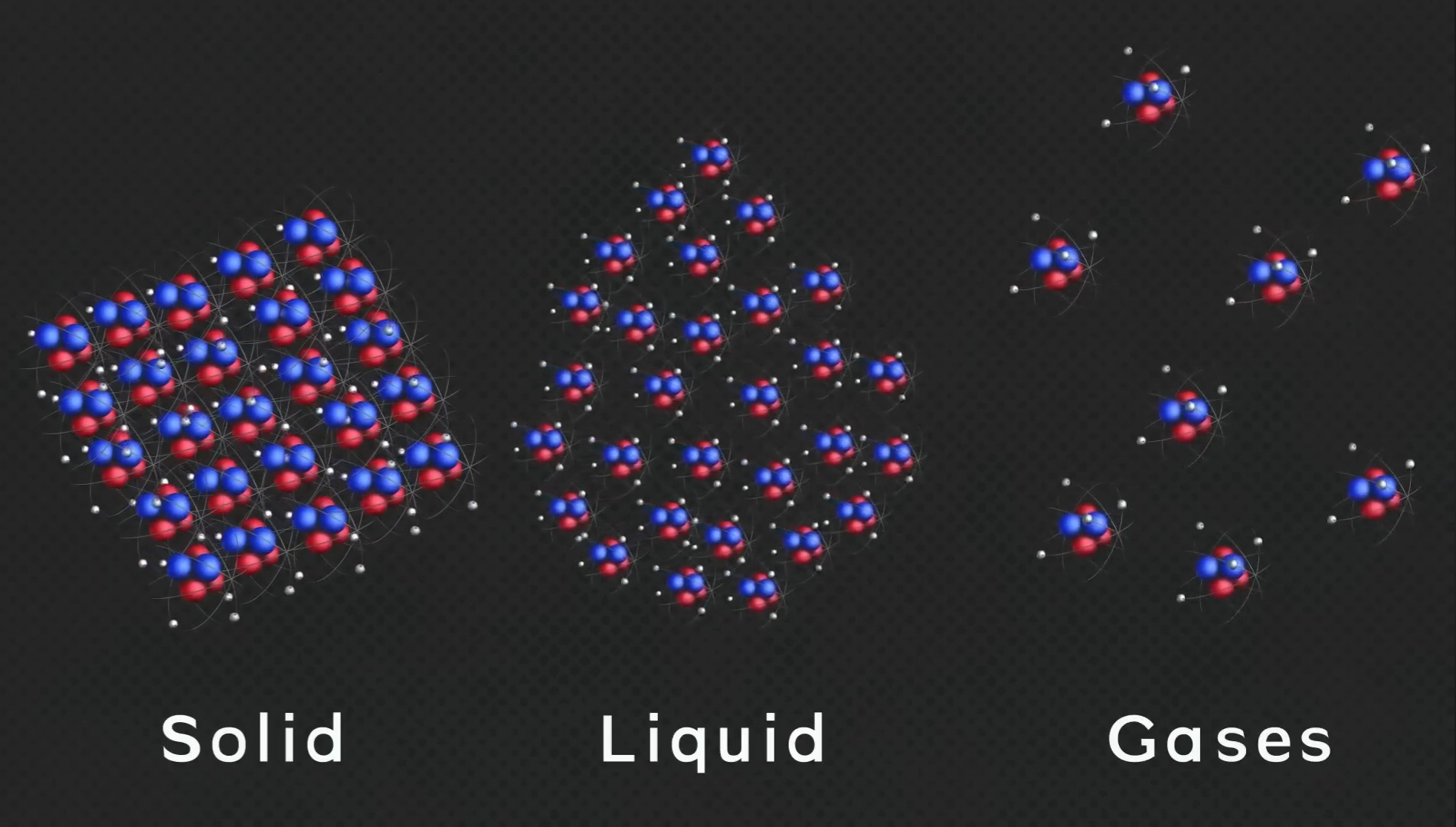
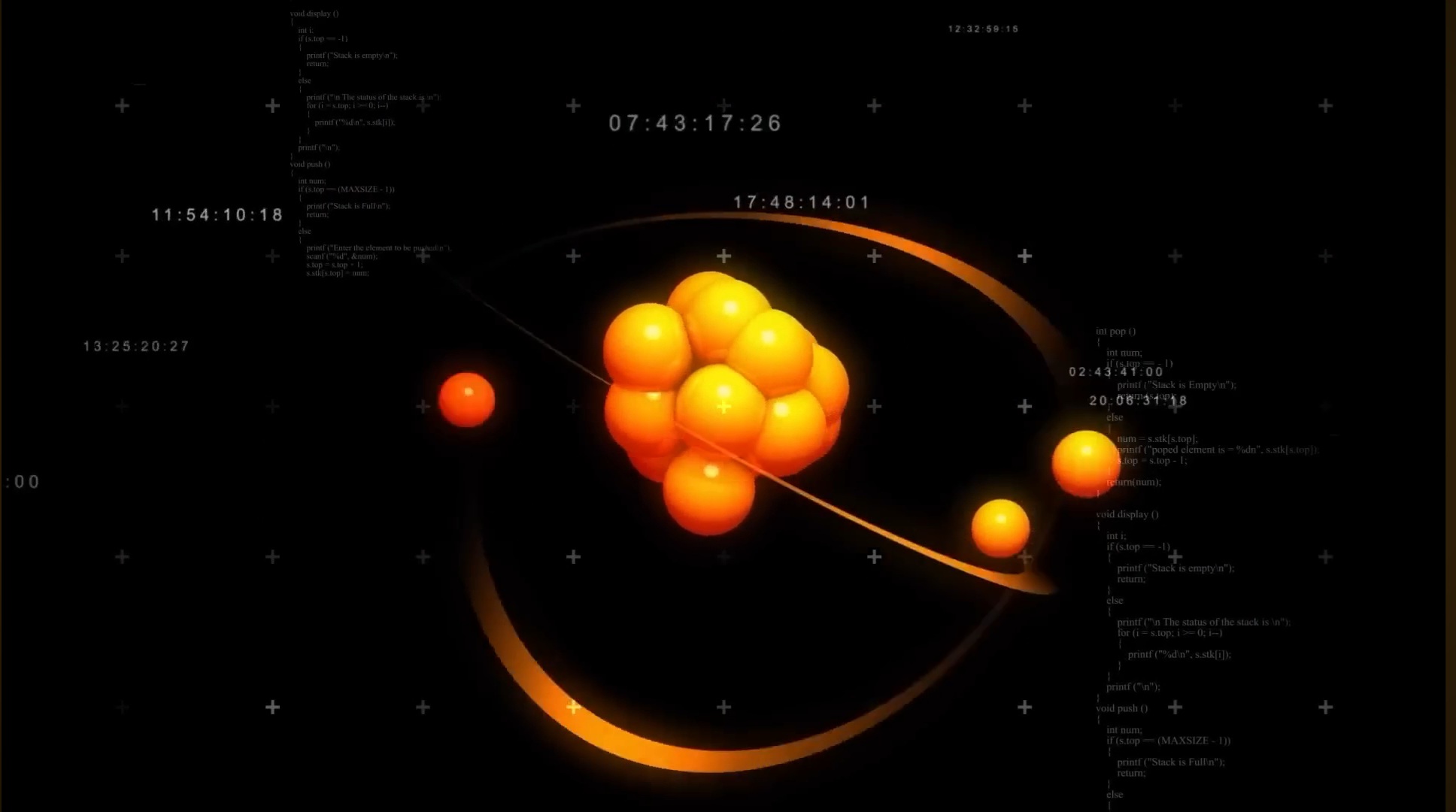
I have a question for you. What are the different states of matter?
I know this might seem like a simple question, so let’s count them:
- Solid
- Liquid
- Gaseous
- Plasma
- Bose-Einstein Condensate
- Degenerate Matter
- Supersolid
- Fermionic Condensates
And the list goes on…

I know that in schools, we are usually taught about just three or, at most, four states of matter. But did you know that there are actually 22 states of matter in the universe?
In fact, one of these states can even defy gravity—and no, I’m not kidding!

Just look at this IMAGE. In it, a liquid is flowing out on its own without any external force being applied. Scientists have named this state Superfluid.
And not just that! Last year, scientists discovered another mysterious state of matter called Quantum Spin Liquid.
Now, these names might sound complex, but understanding these states—and the science behind them—is actually quite easy.
So, are you ready? Let’s begin this journey!
 The Earth’s Magnetic Pole Flip: A Hidden Catastrophe?
The Earth’s Magnetic Pole Flip: A Hidden Catastrophe?
What is Matter?
For years, we have learned that everything around us—like books, computers, food, and even dust on a glass surface—is matter.
By definition, anything that has mass and occupies space is called matter.

And if we talk about the states of matter, we all know that matter is made up of atoms and molecules. The energy within these molecules and their entropy determine the state of that matter.

Suppose I have an ice cube. If we heat it, according to the Second Law of Thermodynamics, the water molecules gain entropy and start converting into liquid.
In simple terms, entropy is increasing.
Similarly, if we keep adding heat, entropy will increase further, and the liquid will turn into a gas.
This is basic school-level physics.
Now, in this process, the molecules themselves do not change—only their entropy does.
But wait a second! If we just talked about the process of increasing entropy, then the reverse should also be possible, right?
We can turn water vapor back into liquid and then freeze the liquid into ice cubes again.
But doesn’t this mean that entropy is decreasing instead of increasing?
Isn’t that a violation of the Second Law of Thermodynamics? How is that even possible?
The Second Law of Thermodynamics Still Holds True
Well, not exactly.

When we convert liquid into ice cubes, the entropy of the liquid decreases.
But at the same time, the energy we supply in this process increases the overall entropy of the universe.
So, in the grand scheme of things, entropy is still increasing, which means the Second Law of Thermodynamics remains valid.
Now, let’s take it a step further.
What if, even after converting into a gaseous state, we continue heating the matter?
 Can the Future Change the Past? The Mystery of Retrocausality and Quantum Mechanics
Can the Future Change the Past? The Mystery of Retrocausality and Quantum Mechanics
The Discovery of Plasma – The Fourth State of Matter

In 1879, an experiment was conducted where gases were heated to such high temperatures that their atoms became so energetic that they ejected their electrons.
Even the protons and neutrons within the nucleus separated.
As a result, a highly excited and extremely energetic soup of protons, neutrons, and free electrons was formed.

This state is known as Plasma.
It sounds exotic, right?
But in reality, this was not the first time we had observed plasma.
We see it all the time in lightning, welding arcs, and even in the Sun.
Yes, that bright star in the sky—the Sun—is nothing but a giant ball of plasma.
In fact, plasma is the most abundant state of matter in the universe.
So now, you understand the fourth state of matter in detail.
Beyond the Fourth State – More States of Matter
As I mentioned earlier, there aren’t just four states—there are a total of 22 states of matter.
But don’t worry! These are just as easy to understand as the four basic ones.
To classify these 22 states, scientists have divided them into two categories:
- Low-temperature states
- High-temperature states
A temperature-pressure diagram helps differentiate between these states.
The Fifth State – Quark-Gluon Plasma

If we heat plasma to an even more extreme temperature, its density and molecular energy increase so much that even protons and neutrons melt.
This results in quarks and gluons moving freely.
This state is called Quark-Gluon Plasma.
It is believed that this state first formed in the universe right after the Big Bang.
While observing this state today is nearly impossible, high-energy nuclear collisions have allowed scientists to study it.
The Sixth State – Degenerate Matter
What happens if we increase pressure instead of temperature?
This leads to Degenerate Matter—an extremely high-density state where pressure does not depend on temperature.
This type of matter is found in white dwarfs and neutron stars.
The Seventh State – Superionic Ice

What if we increase both temperature and pressure at the same time?
This results in Superionic Ice—a type of ice that forms at temperatures of 5000°C and under pressures 2 million times greater than Earth’s atmosphere.
This might sound impossible, but scientists believe such ice exists on planets like Uranus and Neptune.
The Most Recent Discovery – Quantum Spin Liquid

The name Quantum Spin Liquid might make you think it’s some kind of liquid, right?
But you’d be wrong!
This state has nothing to do with liquids.

Instead, it is related to magnetism and how magnets behave at very low temperatures.
Normally, when matter cools down, its electrons align in a particular structure.
However, in Quantum Spin Liquid, electrons continue to move freely, no matter how cold it gets.

This state was first proposed in 1970 by physicist Philip W. Anderson, but it was only proven to exist in 2017.
Does Light Have a State?
We have talked about all these states of matter, but what about light?
Does light have a state?
After all, light is made of photons, and photons are everywhere.
Even your screen is emitting photons right now, allowing you to see this text.
So, doesn’t that mean light occupies space?
And if it occupies space, shouldn’t it be considered a state of matter?
What do you think? Let me know in the comments!
If you learned something new from this post, don’t forget to hit the like button!
As always, friends—
Stay curious, keep learning, and keep growing!